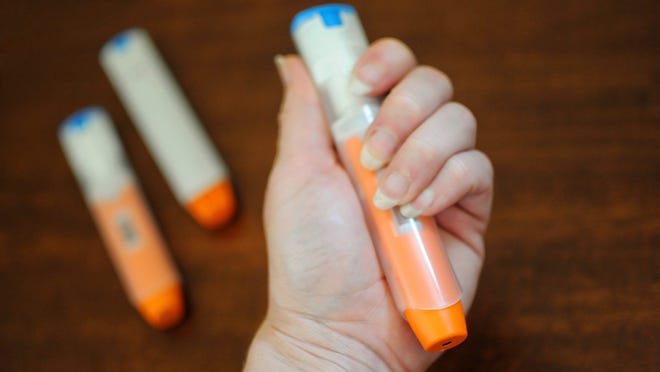
FDA rejects first nasal spray different to epinephrine autoinjectors
The Meals and Drug Administration has declined to approve a substitute for epinephrine autoinjectors resembling EpiPens, telling the maker of a nasal spray that extra examine is required.
ARS Pharma, maker of the drug, stated in an announcement the FDA requested completion of a examine assessing the affect of repeat doses of the spray, known as neffy, in contrast with repeat doses of epinephrine injection. The request comes regardless of an FDA Advisory Committee advice in Might 2023 to approve neffy with out additional examine.
Richard Lowenthal, co-founder and CEO of ARS Pharma, stated the corporate would enchantment the choice however would additionally full the required trial as shortly as attainable.
“We’re very shocked by this motion,” Lowenthal stated. “A number of committee members highlighted the favorable profile of neffy.”
About 32 million folks within the U.S. have meals allergic reactions, based on Meals Allergy Analysis and Training, or FARE, a nonprofit meals allergy advocacy group. Analysis estimates anaphylaxis could trigger as much as 200 deaths every year.
Lowenthal stated neffy will be simply carried and administered “with out anxiousness or hesitation, which is essential to stopping illness development.”
“We’ve heard an incredible outpouring of assist from the affected person, advocacy and doctor communities, who’ve a essential want for a needle-free epinephrine remedy,” he stated.
Dr. Sung Poblete, CEO of FARE, stated the FDA advisory committee really helpful the company approve neffy for adults and kids over 6.
“As one of many greater than 33 million Individuals with life-threatening meals allergic reactions, I’m pissed off by the FDA’s choice at the moment,” Poblete stated in an announcement. “Our neighborhood believed this innovation would lastly come to the greater than 10 % of Individuals with life-threatening meals allergic reactions, however as an alternative, the FDA will pressure us to attend even longer.”

How does neffy work?
There’s no preparation or activation earlier than utilizing neffy, Lowenthal stated. The system works equally to Narcan, the nasal spray that reverses opioid overdoses.
“You seize the system, put it in someone’s nostril and press the plunger on the backside of the system and it’ll snap and spray the medication,” he stated.
The particular person doesn’t must be respiration or snort the treatment, he stated; it’s robotically absorbed by the nasal mucosa.
This is not attainable with regular epinephrine, which is not absorbed within the nostril if taken from a vial. However the brand new nasal spray has a solvent that will get between cells within the nostril and helps the physique take up epinephrine.
Knowledge offered to the FDA exhibits neffy carried out equally to injected epinephrine, stated Dr. Corinna Bowser, an allergist and fellow of the American Academy of Allergy, Bronchial asthma and Immunology, who will not be affiliated with the drug’s research. Injected epinephrine usually produces variable outcomes primarily based on somebody’s age, weight and physique mass, she added.
“What they’re claiming is that (neffy) will not be far more variable or much less variable than among the FDA-approved injectables,” Bowser stated.
Probably the most-reported unintended effects included gentle nasal discomfort, headache, runny nostril, nausea, average dizziness, average vomiting and gentle throat irritation.
Nasal spray wouldn’t require coaching
A 2021 survey revealed discovered within the Annals of Allergy, Bronchial asthma and Immunology discovered epinephrine autoinjectors had been underused in extreme allergic reactions amongst younger sufferers.
Survey respondents stated they didn’t use epinephrine as a result of they didn’t assume the response was extreme sufficient, it was the affected person’s first allergic response, they used different treatment or they had been afraid of utilizing an autoinjector.
Christine Creter stated her 12-year-old son, Colin, should carry injectable types of epinephrine as a result of he has greater than a dozen meals allergic reactions. A nasal spray model can be a lot simpler to manage, she stated.
“I am so disillusioned that our allergic neighborhood has to proceed to attend for a substitute for an injectable type of epinephrine to save lots of our lives,” Creter stated.
Well being specialists hope the nasal spray’s straightforward use means it’ll finally be out there in additional settings: faculties, airplanes and first-aid kits, amongst others. And in contrast to injected epinephrine, no coaching is required, Lowenthal stated.
“Even when you’re skilled to make use of the autoinjector accurately, that doesn’t imply you’ll do it accurately,” stated Poblete. “I’ve heard of oldsters who’ve injected their very own thumb.”
Contributing: Ken Alltucker