Anxiousness drug Clonazepam recalled over mislabeling: See lot numbers
An anxiousness drug is being voluntarily recalled over an error that might be “life-threatening”.
Endo, Inc. issued a voluntary recall for Clonazepam tablets “as a consequence of potential product carton energy mislabeling.” Within the Meals and Drug Administration’s (FDA)recall alert posted on Nov. 19, the pharmaceutical firm is investigating and increasing its beforehand introduced voluntary recall of Clonazepam. The Pennsylvania-based firm issued the primary recall on July 16.

“Endo’s ongoing investigation has recognized the chance that the Clonazepam product heaps listed beneath include a restricted variety of cartons printed with the wrong energy and Nationwide Drug Code (NDC) code as a consequence of an error by a third-party packager,” the FDA’s alert stated.
“The blister strips and tablets contained in the product pack mirror the right energy for the lot,” the alert stated.
As of Nov. 18, when Endo, Inc. issued its recall, the corporate didn’t obtain any studies of opposed occasions associated to the recall.
USA TODAY reached out to Endo, Inc. for remark.
Here’s what you’ll want to know in regards to the Clonazepam recall.
Recall:Prepared-to-eat meat, poultry recalled over listeria danger; 11 infections, 1 loss of life
What’s Clonazepam?
Clonazepam, identified by its model title Klonopin, is a well-liked drug that’s used to deal with some mind and psychological issues.
The oral remedy is usually used to present “a chilled impact on the mind and nerves, which helps to cut back anxiousness, stop seizures, and promote rest”, WebMD explains.
What sorts of Clonazepam are being recalled?
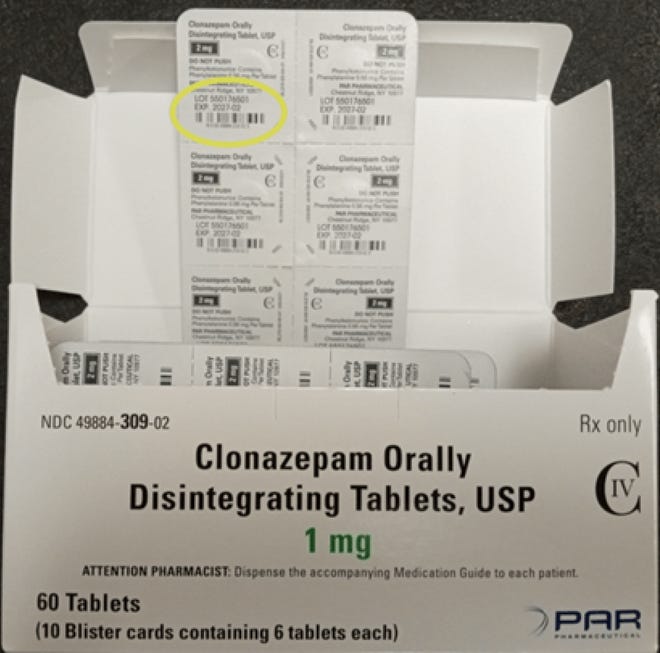
In response to Endo, Inc’s recall, the Clonazepam that’s being voluntarily recalled is the product packaged in cartons containing 60 tablets which are packed into 10 blister strips and include six tablets.

In images offered by Pennsylvania-based firm it reveals the potential mislabeling of a package deal of the Clonazepam tablets, USP 2 mg lot 550176501 with a carton description and NDC code of Clonazepam tablets, USP 1 mg 60-count.
Recalled Clonazepam: See listing of lot numbers
Cannot see the desk? Click on right here to view it.
What are the danger components of the recalled drug?
Endo, Inc. issued the next danger assertion within the recall:
“Kids and adults who inadvertently devour the next dose of clonazepam might be at elevated danger for the opposed occasions of great sedation, confusion, dizziness, diminished reflexes, ataxia, and hypotonia,” the corporate stated.
As well as, the corporate warns of the attainable negative effects for particular sufferers.
“There’s affordable chance for important, presumably life-threatening, respiratory despair particularly for sufferers with concomitant pulmonary illness, sufferers who’ve prescribed dosing close to maximal dosing, and sufferers additionally taking different drugs that would trigger further respiratory despair,” the corporate stated.
What must you do you probably have the recalled drug?
The pharmaceutical firm stated the next precautionary measures needs to be taken you probably have any of the recalled Clonazepam:
- Distributors and retailers ought to cease dishing out the recalled product. It’s extremely really helpful that the product be returned to the place of buy or to contact Inmar.
- Customers are inspired to discontinue utilizing the drug.
- In case you are a client that will have taken an incorrect dose of Clonazepam it’s suggested to contact your physician.
For all questions relating to this recall, you possibly can contact Inmar at 877-890-0765 on Monday by means of Friday from 9 a.m. to five p.m. ET or by e mail at rxrecalls@inmar.com.

Ahjané Forbes is a reporter on the Nationwide Trending Workforce at USA TODAY. Ahjané covers breaking information, automobile recollects, crime, meals recollects, well being, lottery, and public coverage tales. E mail her at aforbes@gannett.com. Comply with her on Instagram, Threads and X (Twitter) @forbesfineest.