Philips to cease US gross sales of sleep apnea units over security issues
Medical system maker Philips will cease promoting sleep apnea units within the U.S. over security issues that might put folks at an elevated threat of most cancers, the corporate introduced Monday as a part of a tentative settlement with federal regulators.
In 2021, the well being expertise firm recalled hundreds of thousands of respiratory units and ventilators globally over issues that foam used to scale back noise and vibration may trigger well being points, together with most cancers.
Phillips has agreed to the phrases of a consent decree stopping U.S. gross sales till required enhancements are made at its vegetation, Philips disclosed in a quarterly earnings replace on Monday. The deal should be permitted by a choose earlier than it’s finalized.

The Dutch producer will proceed servicing the machines already in use underneath the settlement with the U.S. Meals and Drug Administration and Justice Division. The ultimate quantity of the settlement has not been decided however Philips has put aside about $393 million, a number of information companies reported.
In 2022, the FDA ordered the corporate to enhance its outreach to clients concerning the recall and to be extra clear concerning the well being dangers of the merchandise. The company estimated that solely about half the People impacted by the recall had been conscious it had even occurred.
Verify automobile remembers right here:Ford, Tesla, Jaguar amongst practically 2.2 million autos recalled
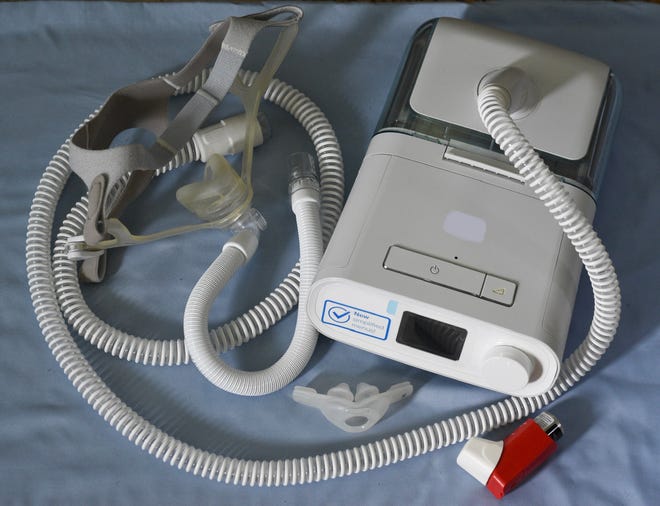
Defect associated to sound foam used to dam noise
Philips introduced a recall for hundreds of thousands of their Bi-Degree Constructive Airway Stress (Bi-Degree PAP), Steady Constructive Airway Stress (CPAP), and mechanical ventilator units in 2021. However “efforts to restore or change the machines have dragged on for years, irritating sufferers within the U.S. and different nations,” reported The Related Press.
The defect was associated to a foam contained in the units that might degrade and trigger customers to breathe in particles and fumes as they sleep.
The corporate has mentioned that there have been no reviews of deaths, however acknowledged that the dangers of particulate publicity may presumably trigger “headache, irritation, irritation, respiratory points and potential poisonous and carcinogenic results.”
The FDA’s web site says that ingesting the sound-dampening foam comes with the dangers of headache, bronchial asthma, allergic reactions amongst extra critical issues. The company additionally warned in November that the machines can overheat and in uncommon circumstances trigger fires.
CEO guarantees security and accountability
In a submitting with the Securities and Change Fee, Royal Philips CEO Roy Jakobs promised that the corporate prioritizes affected person security and high quality.
“Resolving the implications of the … recall for our sufferers and clients is a key focus space and I acknowledge and apologize for the misery and concern brought on. We’re absolutely dedicated to complying with the consent decree, which is a vital step and offers a transparent path ahead,” Jakobs mentioned.